Abstract
Background: Angioimmunoblastic T cell lymphoma (AITL) is one of the most frequent peripheral T cell lymphoma, and has a poor prognosis. Neoplastic T cells originate from T follicular helper cells, and are admixed among a prominent microenvironment, making their identification sometimes difficult. IDH2 mutations are present in 20-30% AITL patients, where they often co-exist with TET2, DNMT3A and RHOA mutations. They affect almost exclusively the codon R172 of IDH2, providing to the IDH2 enzyme a neo-activity that converts α ketoglutarate (αKG) to D 2-hydroxyglutarate (2HG). D-2HG, the dextrogyre form of 2HG, is an oncometabolite present at very low level under physiological condition, which inhibits many αKG dependent dioxygenases, including TET proteins and is involved in oncogenesis of various cancers such as gliomas or acute myeloid leukemias (AML). Preliminary data, based on small series, showed that increased level of 2HG was detectable in tumor and in serum of IDH2 mutated AITL. However, 2HG level, as well as D/L ratio, has not been evaluated as a surrogate marker of IDH2 mutation in AITL, at diagnosis or during the follow-up.
Patients and Methods: Serum from 69 AITL patients, collected in REVAIL trial (NCT00169156) (N=48), RAIL trial (NCT01553786) (N=9) or TENOMIC collection (N=12) were included in this study. IDH2 mutations were assessed in formalin fixed paraffin embedded tumor tissue by deep next generation sequencing of exon 4, using PGM technology (N=63) or allele specific PCR (N=6). For the purpose of the study the cohort was enriched in mutated patients. Serum was collected at diagnosis and at the end of the frontline treatment in 6 patients, 5 of them being IDH2 mutated. D and L 2HG was measured in serum using a liquid tandem mass spectrometry method as previously described (Poinsignon et al. J Chromatogr B 2016) to determine total (D+L) 2HG and D/L 2HG ratio.
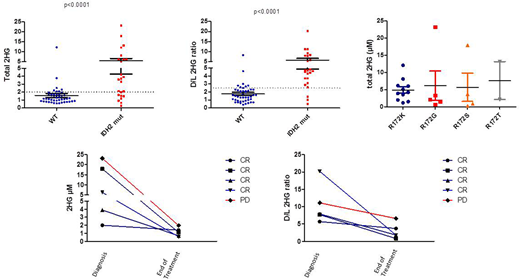
Results: Twenty-four patients (35%) were IDH2 mutated. Median IDH2 variant allele frequency (VAF) was 7% (IQR, 4%-12.5%). Median total 2HG was 3.63 µM (IQR, 1.6-6.1) in mutated patients versus 1.17 µM (IQR, 0.85-1.68) in wild type patients (p<0.001) (figure). D/L was also found significantly higher in mutated [4.7 (IQR 3.52-7.74)] than in WT patients [1.5 (IQR 0.94-2.23)] (p<0.001). IDH2 mutations included 11 IDH2R172K mutations, 5 IDH2R172G mutations, 4 IDH2R172S, 2 IDH2R172T, 1 IDH2R172M and 1 IDH2R140Q, all showing similar level of 2HG, without variation of 2HG level depending on the mutation. 2HG level correlated with IDH2 mutation VAF (Spearman r=0.4590, p=0.024) likely paralleling the tumor cells mass. Using the cut off established in AML, 2µM and 2.5 for respectively total 2HG and D/L ratio, we found that 17/24 (71%) mutated patients versus 7/45 (15%) had a total 2HG ≥ 2, and that 21/24 (87.5%) mutated versus 8/45 (18%) WT patients had D/L 2HG ratio ≥2.5, leading to a sensibility of 71% and 84%, and a specificity of 87% and 82%, respectively for total 2HG and D/L ratio to detect IDH2 mutations. End of treatment 2HG dosage was available in 5 IDH2 mutated patients with elevated 2HG at diagnosis. All patients had a total 2HG decreased below 2µM. Three patients normalized their D/L ratio, 2 of them being in complete response and one being not evaluable. In contrary, two patients kept a D/L 2HG ratio >2.5, one having progressive disease while the other remains in complete response 6 months after the end of treatment.
Conclusion: Serum total 2HG and D/L 2HG ratio can reliably predict the presence of IDH2 mutation in AITL patients. Most patients in complete remission normalize their dosage and ratio, suggesting that serum 2HG could be a marker of residual disease that could be used to monitor patients. Specific cut-off values of 2HG and D/L 2HG ratio for AITL are under investigation.
Ribrag:Servier: Consultancy, Honoraria; pharmamar: Other: travel; Gilead: Consultancy, Honoraria; Amgen: Research Funding; epizyme: Consultancy, Honoraria; Roche: Honoraria, Other: travel; argenX: Research Funding; NanoString Technologies: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: travel; Incyte Corporation: Consultancy; MSD: Honoraria; Infinity: Consultancy, Honoraria. Tilly:Astra-Zeneca: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria. Haioun:Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Sciences: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Gaulard:Roche: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal